- Reproxalap Statistically Lower than Xiidra for the Two Endpoints of the Trial: Ocular Discomfort (p=0.002) and Ocular Itching (p=0.01)
- Results Consistent with Data from Post-Acute Ocular Tolerability Comparison of Reproxalap and Xiidra in Patients with Dry Eye Disease
Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company discovering and developing innovative therapies for the treatment of immune-mediated diseases, today announced positive top-line data from a Phase 2 clinical trial comparing ocular discomfort and itching symptom scores of reproxalap ophthalmic solution 0.25% (reproxalap), an investigational new drug, versus Xiidra® (lifitegrast ophthalmic solution 5%) in patients with dry eye disease. Patient-reported ocular discomfort (p=0.002) and itching (p=0.01) were statistically lower with reproxalap than with Xiidra.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220110006109/en/
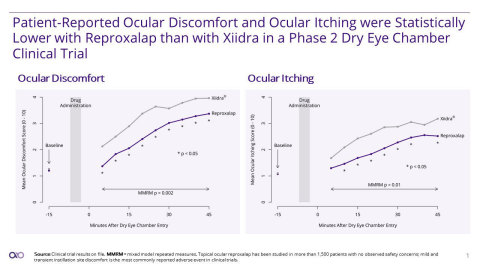
Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) on January 11, 2022 announced positive top-line data from a Phase 2 clinical trial comparing ocular discomfort and itching symptom scores of reproxalap ophthalmic solution 0.25% (reproxalap), an investigational new drug, versus Xiidra® (lifitegrast ophthalmic solution 5%) in patients with dry eye disease. Patient-reported ocular discomfort (p=0.002) and itching (p=0.01) were statistically lower with reproxalap than with Xiidra®. (Graphic: Aldeyra Therapeutics)
“The statistically significant symptom improvement of reproxalap over Xiidra observed in this trial reinforces the data from an earlier clinical trial demonstrating tolerability advantages of reproxalap over Xiidra in patients with dry eye disease,”1 stated Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer of Aldeyra. “The combination of rapid activity and improved tolerability evidenced by reproxalap in clinical testing has the potential to address significant compliance issues with currently available therapy, the median discontinuation rates of which are approximately one month.”2
“The clinically relevant and statistically significant reduction in symptomatic activity suggest that reproxalap, if approved, has the potential to become a first-line therapy for the treatment of dry eye disease,” stated Peter Couroux, M.D., Global Senior Medical Director for Cliantha Research and the principal investigator of the clinical trial. “As the first RASP modulator developed for ocular surface disease, reproxalap represents a new mechanism of action that may expand the treatment options available to dry eye disease patients.”
The double-masked, crossover, single-center, Phase 2 clinical trial in 56 dry eye disease patients evaluated the activity of reproxalap compared to Xiidra for two endpoints: ocular discomfort symptom score and ocular itching symptom score. A single dose of test article was administered to both eyes approximately 5 minutes prior to a dry eye chamber exposure lasting 45 minutes, during which humidity was maintained at low levels in a setting of regulated air flow, temperature, and visual tasking. Symptoms were assessed approximately 15 minutes prior to chamber entry, and every 5 minutes beginning 5 minutes after chamber entry.
No safety signals were observed in the trial, and there were no treatment-related discontinuations or moderate or serious adverse events related to drug. The most common adverse event in both treatment arms was mild instillation site discomfort. Reproxalap has now been evaluated in more than 1,500 patients. The approved commercial dosing regimen of Xiidra and the intended commercial dosing regimen of reproxalap include repeated administration of drug.
Aldeyra plans to present data from the trial at an upcoming medical meeting.
About Reproxalap
Reproxalap, an investigational new drug, is a novel small-molecule modulator of RASP (reactive aldehyde species), which are elevated in ocular and systemic inflammatory disease. Reproxalap’s mechanism of action has been supported by the demonstration of statistically significant and clinically relevant activity in multiple physiologically distinct late-phase clinical indications. Reproxalap is currently in Phase 3 clinical development as a 0.25% ophthalmic solution for the treatment of dry eye disease and allergic conjunctivitis, two of the largest markets in ophthalmology.
About Dry Eye Disease
Dry eye disease is a common inflammatory disease estimated to affect 34 million or more adults in the United States.3 The disease is characterized by insufficient lubrication of the anterior surface of the eye, leading to dryness, inflammation, pain, discomfort, irritation, diminished quality of life, and in severe cases, permanent vision impairment. Among many physicians and patients, existing therapy for dry eye disease is generally regarded as inadequate and often requires weeks or months to demonstrate activity. In patients with dry eye disease, pro-inflammatory RASP may contribute to ocular inflammation and changes in tear lipid composition.4 By diminishing RASP levels, Aldeyra’s lead RASP modulator reproxalap represents a novel and differentiated approach for the treatment of the symptoms and signs of dry eye disease.
About Aldeyra Therapeutics
Aldeyra Therapeutics discovers and develops innovative therapies designed to treat immune-mediated diseases. Our approach is to develop therapies that modulate immunological systems, instead of inhibiting or activating single targets, with the goal of optimizing multiple pathways at once while minimizing toxicity. Two of our lead product candidates, reproxalap and ADX-629, target pre-cytokine, systems-based mediators of inflammation known as RASP (reactive aldehyde species). Reproxalap is in Phase 3 clinical trials in patients with dry eye disease and allergic conjunctivitis. ADX-629, an orally administered RASP modulator, is in Phase 2 proof-of-concept clinical trials in psoriasis, asthma, and COVID-19. Our pipeline also includes ADX-2191 (intravitreal methotrexate 0.8%), in development for the prevention of proliferative vitreoretinopathy and the treatment of retinitis pigmentosa and primary vitreoretinal lymphoma. For more information, visit https://www.aldeyra.com/ and follow us on LinkedIn, Facebook, and Twitter.
Safe Harbor Statement
This release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding Aldeyra's strategy, future operations, prospects, plans, and objectives and Aldeyra's plans and expectations for its product candidates, including reproxalap. Aldeyra intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by terms such as, but not limited to, "may," "might," "will," "objective," "intend," "should," "could," "can," "would," "expect," "believe," "anticipate," "project," "on track," "scheduled," "target," "design," "estimate," "predict," "potential," "aim," "plan" or the negative of these terms, and similar expressions intended to identify forward-looking statements. Such forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions, and uncertainties. Aldeyra is at an early stage of development and may not ever have any products that generate significant revenue. All of Aldeyra's development timelines may be subject to adjustment depending on recruitment rate, regulatory review, preclinical and clinical results, and other factors that could delay the initiation or completion of clinical trials. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements include, among others, the timing of enrollment, commencement and completion of Aldeyra's clinical trials; the timing and success of preclinical studies and clinical trials conducted by Aldeyra and its development partners; updated or refined data based on Aldeyra's continuing review and quality control analysis of clinical data, Aldeyra's ability to design clinical trials with protocols and endpoints acceptable to applicable regulatory authorities; delay in or failure to obtain regulatory approval of Aldeyra's product candidates; the ability to maintain regulatory approval of Aldeyra's product candidates, and the labeling for any approved products; the risk that prior results, such as signals of safety, activity or durability of effect, observed from preclinical or clinical trials, will not be replicated or will not continue in ongoing or future studies or clinical trials involving Aldeyra's product candidates in clinical trials focused on the same or on different indications; the risk that the results from earlier clinical trials, portions of clinical trials, or pooled clinical data may not accurately predict results of subsequent trials or the remainder of a clinical trial; the risk that results from comparative clinical trials, such as ocular discomfort and itching comparisons, may not be replicated with repeated administration of drug as directed by the intended commercial dosing regimen and the commercial dosing regimen, as applicable; the scope, progress, expansion, and costs of developing and commercializing Aldeyra's product candidates; uncertainty as to Aldeyra’s ability to commercialize (alone or with others) Aldeyra's product candidates following regulatory approval, if any; the size and growth of the potential markets and pricing for Aldeyra's product candidates and the ability to serve those markets; Aldeyra's expectations regarding Aldeyra's expenses and revenue, the sufficiency or use of Aldeyra's cash resources and needs for additional financing; political, economic, legal, social and health risks, including the COVID-19 pandemic and related public health measures, that may affect Aldeyra’s business or the global economy; the rate and degree of market acceptance of any of Aldeyra's product candidates; Aldeyra's expectations regarding competition; Aldeyra's anticipated growth strategies; Aldeyra's ability to attract or retain key personnel; Aldeyra’s limited sales and marketing infrastructure; Aldeyra's ability to establish and maintain development partnerships; Aldeyra’s ability to successfully integrate acquisitions into its business; Aldeyra's expectations regarding federal, state and foreign regulatory requirements; regulatory developments in the United States and foreign countries; Aldeyra's ability to obtain and maintain intellectual property protection for its product candidates; the anticipated trends and challenges in Aldeyra's business and the market in which it operates; and other factors that are described in the "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of Aldeyra's Annual Report on Form 10-K for the year ended December 31, 2020 and Quarterly Report on Form 10-Q for the quarter ended September 30, 2021, which are on file with the Securities and Exchange Commission (SEC) and available on the SEC's website at https://www.sec.gov/. Additional factors may be described in those sections of Aldeyra's Annual Report on Form 10-K for the year ended December 31, 2021, expected to be filed with the SEC in the first quarter of 2022.
In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra's results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. The information in this release is provided only as of the date of this release, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this release on account of new information, future events, or otherwise, except as required by law.
1 McMullin D, Clark D, Cavanagh B, Karpecki P, Brady TC. A Post-Acute Ocular Tolerability Comparison of Topical Reproxalap 0.25% and Lifitegrast 5% in Patients with Dry Eye Disease. Clin Ophthalmol. 2021 Sep 22;15:3889-3900.
2 White DE, Zhao Y, Ogundele A, Fulcher N, Acs A, Moore-Schiltz L, Karpecki PM. Real-World Treatment Patterns Of Cyclosporine Ophthalmic Emulsion And Lifitegrast Ophthalmic Solution Among Patients With Dry Eye. Clin Ophthalmol. 2019 Nov 22;13:2285-2292.
3 Paulsen AJ, Cruickshanks KJ, Fischer ME, Huang GH, Klein BE, Klein R, Dalton DS. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014 Apr;157(4):799-806. doi: 10.1016/j.ajo.2013.12.023. Epub 2014 Jan 2. PMID: 24388838; PMCID: PMC3995164.
4 Choi W, Lian C, Ying L, Kim GE, You IC, Park SH, Yoon KC. Expression of Lipid Peroxidation Markers in the Tear Film and Ocular Surface of Patients with Non-Sjogren Syndrome: Potential Biomarkers for Dry Eye Disease. Curr Eye Res. 2016 Sep;41(9):1143-9. doi: 10.3109/02713683.2015.1098707. Epub 2016 Jan 5. PMID: 26731289.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220110006109/en/
Contacts
Corporate:
Joshua Reed
Aldeyra Therapeutics, Inc.
Tel: 781-761-4904 ext. 218
jreed@aldeyra.com
Investors & Media:
Scott Solomon
Sharon Merrill Associates, Inc.
Tel: 617-542-5300
ALDX@investorrelations.com







