Wireless Smart Neurostimulation System records from 10× more brain sites than current adaptive implants; a large-animal study demonstrates chronic sensing, neural-state decoding, and programmable stimulation
Nia Therapeutics announced publication in Brain Stimulation of the first in vivo validation of its Smart Neurostimulation System (SNS), a wireless, implantable brain-computer interface designed for closed-loop treatment of memory disorders.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20260102654553/en/
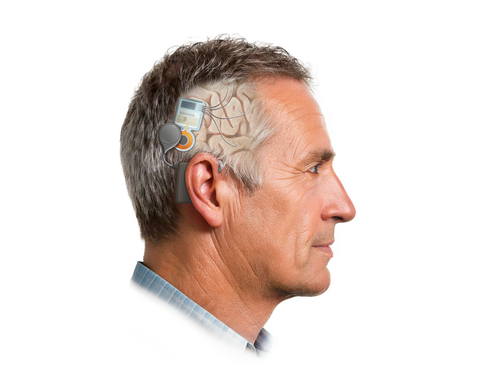
The SNS records neural activity from 60 channels across four brain regions—an order-of-magnitude increase over commercially available devices. The NeuroPace RNS system, FDA-cleared for epilepsy, records from up to six channels; Medtronic's Percept adaptive DBS system records from up to four.
This expanded sensing capacity reflects the distributed nature of memory, which arises from coordinated dynamics across widespread neural networks rather than from a single focal site.
"Most brain implants were developed for conditions in which a localized abnormal signal drives symptoms," said Michael J. Kahana, PhD, co-founder and CEO of Nia Therapeutics and a professor at the University of Pennsylvania. "Decades of research show that memory depends on coordinated activity across distributed networks. The SNS was engineered to detect these patterns and respond with personalized stimulation."
Preclinical Validation in Freely Moving Large Animals
In a chronic study of three sheep, the SNS demonstrated stable performance across core functions:
- Neural-state decoding. Machine-learning classifiers distinguished movement from stillness with high accuracy (AUC 0.92–0.98), with performance stable throughout implantation.
- Programmable neuromodulation. Systematic variation of stimulation parameters produced dose-dependent changes in alpha-band (8–12 Hz) and gamma-band (78–82 Hz) neural activity, confirming that stimulation reliably modulates physiological signals.
- Biocompatibility. Histological analyses showed no adverse tissue response, with findings comparable to a commercially available control lead.
These results demonstrate that the SNS can chronically record distributed neural activity, decode behaviorally relevant brain states, and deliver stimulation with predictable effects—key prerequisites for future closed-loop neurostimulation therapies.
Building on a Decade of Human Memory Research
The SNS builds on federally funded research supported by DARPA and NIH. In prior human studies using externalized research systems, Kahana and colleagues recorded intracranial brain activity from hundreds of epilepsy patients performing memory tasks and showed that machine-learning models could predict, moment by moment, whether newly learned information would be remembered.
In sham-controlled clinical experiments, brief bursts of electrical stimulation delivered during classifier-identified poor-encoding states improved delayed recall by approximately 20%; stimulation delivered at random times produced no benefit. Those studies established the therapeutic principle underlying Nia's approach but relied on devices unsuitable for chronic use.
"This publication shows that the core capabilities required for memory-guided stimulation—high-density sensing, real-time decoding, and programmable neuromodulation—can be delivered in a fully implantable, wireless system," said Daniel S. Rizzuto, PhD, co-founder and President of Nia Therapeutics.
Addressing an Unmet Clinical Need
Memory impairment is among the most common and disabling consequences of traumatic brain injury and age-related cognitive decline. Although recent disease-modifying drugs for early Alzheimer's can slow progression, they do not restore lost function. Nia's approach aims to complement such treatments by directly improving memory through targeted neuromodulation.
Nia Therapeutics is preparing for first-in-human studies, with regulatory submissions planned for 2026. The initial study will focus on patients with memory loss resulting from moderate-to-severe traumatic brain injury.
About Nia Therapeutics
Nia Therapeutics develops implantable brain-computer interfaces for memory disorders. Founded in 2018, the company's SNS device supports closed-loop neuromodulation by detecting brain states linked to impaired memory encoding and delivering targeted stimulation. Visit www.niatx.com.
Publication Reference
Rizzuto DS, Herrema HG, Hu Z, Utin D, Kahn J, Ho C, Smiles A, Gross RE, Lega BC, Das SR, Kahana MJ. A wireless, 60-channel, AI-enabled neurostimulation platform. Brain Stimulation (2025). DOI: https://doi.org/10.1016/j.brs.2025.103013
View source version on businesswire.com: https://www.businesswire.com/news/home/20260102654553/en/
Contacts
Media Contact
Michael Kahana, PhD, CEO, Nia Therapeutics, mike@niatx.com







