CAMBRIDGE, England - February 12, 2020 - (Newswire.com)
Emerging pathogens, such as Ebola, Zika, Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV) are becoming a growing threat due to urbanisation, climate change and the global migration of people. The current outbreak of a novel coronavirus (2019-nCoV) has hit China in the past month and spread to 25 nations. It has affected more than 42,000 people and cost more than 1,000 lives at the point when this article is being processed. The global economic loss is expected to be over $40 billion, larger than that suffered during the SARS outbreak in 2003.
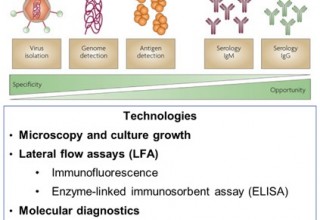
Early detection and diagnosis are essential to reduce the prevalence of infectious diseases and to prevent them from becoming epidemic or even pandemic. What are the methods available to detect infectious diseases? Figure 1 summarises the methods currently available.
Traditionally, pathologists have used culture growth and microscopy. However, it usually takes several days to grow bacteria in medium and it's not suitable for detecting viruses due to their small size and slow growth. In a fast-paced medical world, an immediate and accurate diagnosis and quicker methods are necessary. A faster and cheaper method would be lateral flow assays (LFA), such as immunoassays (using antibodies) or enzyme-linked immunosorbent assay (ELISA). Such a device is similar to a pregnancy test strip and does not require expensive equipment. However, this method hardly works for the new coronavirus disease (2019-nCoV) due to the limitations of the non-specification, low-sensitivity and long development process (up to three months).
For the rapid detection of coronavirus (2019-nCoV), however, molecular diagnostics (MDx) is the best, if not the only, option at the moment. MDx consists of diagnosis at the molecular level, identifying the DNA or RNA of a pathogen. This is a fast-changing field driven by two trends: DNA chips or PCR enabled point-of-care MDx and the next-generation DNA sequencing. In this article, IDTechEx discusses how the PCR technique is being used to tackle the coronavirus diagnosis. This article is drawn from “Molecular Diagnostics 2020-2030” which offers the full landscape of the molecular diagnostic market, analysis of players, assessment of innovations, emerging market and a 10-year market forecast.
How Molecular Diagnostics is helping fight the spread of coronavirus virus
In December 2019, Chinese scientists identified the RNA sequence of the 2019-nCoV by the whole genome sequencing method, classifying 2019-nCoV as a positive-sense RNA virus. Based on the sequence, the first Coronavirus detection kit was developed by a group of researchers from the German Center for Infection Research (DZIF) at Charité Hospital in Berlin.
This world’s first diagnostic test for the coronavirus is based on reverse transcription polymerase chain reaction (RT-PCR) and has now been made publicly available following its online publication by the World Health Organisation (WHO). More than 52 companies have now developed analogous diagnostic kits within weeks in China. Seven of them have been approved by the National Medical Products Administration (NMPA) and received a medical device registration certificate, as listed in table 1. So far, the dominant method is real-time PT-PCR. Other methods based on DNA microarrays are under development.
Before using the MDx kit to detect the 2019-nCoV, the sample needed to be treated following the procedure outlined in Figure 2. However, now, once collected, the sample is then transferred to a central lab where technical staff will extract and purify the RNA before adding to the 2019-nCoV diagnostic kit. The suppliers of the reagents and systems for the extraction and purification include ThermoFisher, Qiagen, bioMérieux and Roche. Many of these companies are providing ready-to-use RT-PCR workflow products to tackle the 2019-nCoV outbreak. To learn more key players in different steps of MDx, please go to the IDTechEx report “Molecular Diagnostics 2020-2030”.
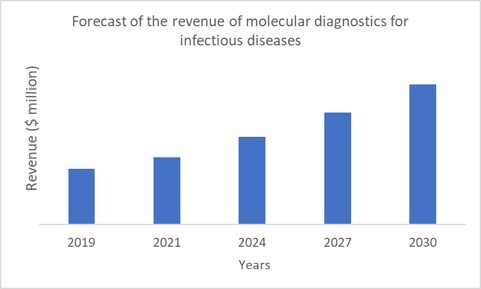
It is a still on-going battle to fight against the 2019-nCoV and the public is increasingly aware of the big threat of the spread of infectious diseases. As traditional diagnosis fails to cope with novel pathogens and transmission of pathogens becomes faster, molecular diagnostics will rise to sit at the core of prevention of pandemic tragedies. Indeed, MDx is one of the fastest-growing markets in the healthcare industry, driven by the fast-developing technologies such as the third-generation DNA sequencing, digital PCR and microfluidic devices. IDTechEx forecasts MDx for infectious disease detection will become a $7 billion USD market by 2030.
The new “Molecular Diagnostics 2020-2030” report covers the technologies, benchmarking, and players of DNA arrays, PCR devices and DNA sequencing from the hardware. The report highlights the recent innovations and trends in digital PCR, third-generation DNA sequencing and more. It also provides a 10-years forecast on each technology segment and end-user market including infectious disease, cancer, liquid biopsy, genetic testing, companion diagnostic and beyond.
To find out more about Life Sciences research available from IDTechEx visit www.IDTechEx.com/LifeSci or to connect with others on this topic, IDTechEx Events is hosting: Healthcare Sensor Innovations 2020 Conference on March 17-18, 2020 in San Jose, USA. Please visit www.HealthcareSensorInnovations.com/USA.
IDTechEx guides your strategic business decisions through its Research, Consultancy and Event products, helping you profit from emerging technologies. For more information on IDTechEx Research and Consultancy contact research@IDTechEx.com or visit www.IDTechEx.com.
Media Contact:
Jessica Abineri
Marketing Coordinator
press@IDTechEx.com
+44(0)1223 812300
Related Links
Further IDTechEx Research on Life Sciences
Healthcare Sensor Innovations 2020 | 17 - 18 March 2020 | San Jose, USA
Related Images
Press Release Service by Newswire.com
Original Source: Molecular Diagnostics Sits at Heart of the Fight Against the Coronavirus, According to New IDTechEx Report

